Welcome micromolar a molar to the world of micromolars and molars, where tiny concentrations hold immense significance in scientific research! If you’ve ever found yourself scratching your head at these seemingly complex units, fret not. In this blog post, we’ll unravel the mysteries behind micromolar and molar measurements, empowering you to make accurate calculations like a true scientist. So prepare to dive deep into the realm of concentration units and discover how they can revolutionize your research game. Let’s get started on this exciting journey together!
What are Micromolars and Molars?
Micromolars and molars are concentration units used in scientific measurements to quantify the amount of a substance dissolved in a solution. These units play a crucial role in various fields such as biochemistry, pharmacology, and environmental science.
To understand micromolars and molars, we need to first grasp the concept of molarity. Molarity refers to the number of moles of solute present per liter of solution. It is denoted by “M” or “mol/L.” A micromolar (μM) is one-thousandth of a millimolar (mM), which is equivalent to 10^-6 moles per liter.
On the other hand, a molar (M) represents an even higher concentration unit with one mole of solute dissolved in one liter of solution. In simpler terms, it signifies a more concentrated solution compared to micromolar.
These two units provide researchers with precise measurements for studying reactions, concentrations, and reactions rates accurately. Whether you’re analyzing enzyme activity or determining drug dosage levels, understanding micromolars and molars is essential for accurate calculations and analysis.
Now that we have acquainted ourselves with these concentration units let’s delve deeper into their importance within scientific research!
Understanding the Importance of Concentration Units
Understanding the Importance of Concentration Units
When it comes to scientific research and experiments, accurately measuring concentrations is crucial. Concentration units provide a standardized way to express the amount of a substance dissolved in a solution. They help scientists compare results, replicate experiments, and draw meaningful conclusions.
One common concentration unit used in science is molar (M). Molar concentration measures the number of moles of solute per liter of solution. It provides information about the number of particles present in a given volume and allows for precise calculations and comparisons.
Another widely used concentration unit is micromolar (µM), which represents one millionth of a molar. Micromolars are often used when dealing with very small quantities or low concentrations of substances.
Choosing the appropriate concentration unit depends on various factors like the nature and purpose of your experiment, as well as equipment limitations. Using an incorrect unit can lead to inaccuracies or misinterpretations in your data analysis.
Accurate measurement requires attention to detail and proper calibration techniques. Instruments like spectrophotometers or pipettes must be calibrated regularly to ensure reliable measurements at specific concentration levels.
In addition to accuracy, it’s important to consider practicality when selecting a concentration unit. For instance, using micromolars might be more suitable if you’re working with rare or expensive substances that require careful conservation.
Understanding the importance of concentration units lays the foundation for successful scientific research. By choosing the right unit and employing accurate measurement techniques, scientists can obtain reliable data that contributes valuable insights into their field.
Converting Between Micromolar and Molar
Converting between micromolar (µM) and molar (M) concentrations is a fundamental skill in scientific research. Whether you’re studying chemical reactions or working with biological samples, being able to accurately convert between these concentration units is crucial.
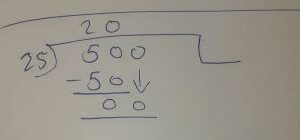
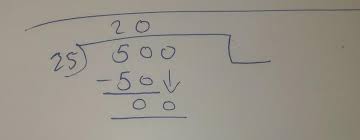
To convert from micromolar to molar, divide the given value by 1,000,000. For example, if you have a solution with a concentration of 500 µM, divide it by 1,000,000 to obtain the equivalent value in Molarity – which would be 0.5 M.
On the other hand, if you want to convert from molar to micromolar just multiply the given value by 1,000,000. So if you have a solution with a concentration of 2 Molarity and need it in micromolars instead – simply multiply it by 1 million and voila! The result will be 2 million µM.
Remember that accurate measurements are vital when converting between these units. Always double-check your calculations and pay attention to significant figures. Additionally,
be mindful of any dilutions or volume changes that may affect your final concentration calculation.
Mastering this conversion process will benefit scientists across various disciplines – from chemists analyzing reaction kinetics to biologists determining enzyme activity levels.
The ability to seamlessly switch between micromolars and molars enhances experimental precision and enables researchers to compare data across studies more easily.
While understanding how different concentrations relate can greatly facilitate scientific experiments,
it’s important not only know how but also when
to use each unit effectively.
Choosing the appropriate concentration unit for your research needs ensures accuracy while providing clear communication within the scientific community.
In summary,
the ability to convert between micromolars and molars is essential for any scientist working with solutions or substances of varying concentrations.
By mastering this skill,
researchers can ensure accurate measurements,
compare data effectively,
and contribute meaningfully
to their field of study.
Tips for Accurate Micromolar and Molar Measurements
When it comes to accurate micromolar and molar measurements, there are a few tips that can help ensure precise results. It is important to calibrate your instruments regularly. This will help maintain accuracy and account for any variations that may occur over time.
Another tip is to carefully handle and prepare your samples. Contamination or improper dilution can lead to inaccurate measurements. Be sure to follow proper protocols and use clean equipment when working with solutions.
Additionally, it is crucial to pay attention to temperature during measurements. The solubility of compounds can vary with temperature, so maintaining a consistent temperature throughout the experiment will improve accuracy.
It’s also important to note the pH of the solution being measured. Some compounds may exhibit different behaviors at certain pH levels, so adjusting the pH if necessary can provide more accurate results.
Always double-check your calculations and record data meticulously. Small errors in concentration calculations or recording values can have significant impacts on your final results.
By following these tips for accurate micromolar and molar measurements, you can increase the reliability of your research findings in various scientific fields such as chemistry, biology, and pharmacology.
Common Applications of Micromolar and Molar in Science
Common Applications of Micromolar and Molar in Science
Micromolar (μM) and molar (M) concentrations are widely used in various scientific disciplines for a range of applications. One common application is in biochemistry and molecular biology experiments, where researchers often need to determine the concentration of a particular substance such as enzymes or small molecules.
In drug discovery research, micromolars and molar units are crucial for assessing the potency of potential therapeutic compounds. By measuring the concentration required to achieve a desired biological effect, scientists can evaluate the efficacy of different drug candidates.
Additionally, these units play a vital role in analytical chemistry techniques like spectrophotometry and chromatography. These methods rely on precise measurements of concentrations to determine components present in complex mixtures or quantify specific analytes.
Micromolars and molar units also find extensive use in cell culture studies. Researchers often need to prepare solutions with specific concentrations to maintain cells under optimal conditions or induce certain experimental treatments.
Moreover, micromoles per liter (µmol/L) is frequently employed in clinical diagnostics when measuring blood markers or hormone levels. This allows healthcare professionals to assess patients’ health status accurately and detect any abnormalities that may require further investigation or treatment.
Micromolars and molar units provide essential tools for scientists across various fields by enabling accurate quantification of substances involved in experiments, drug development, chemical analysis, cell culture maintenance, and clinical diagnostics. Their widespread applicability highlights their significance as fundamental measurement units within the scientific community.
Advantages and Limitations of Using These Units
Advantages and Limitations of Using These Units
Using micromolar and molar units in scientific research offers several advantages. These concentration units provide a standardized way to express the amount of a substance dissolved in a given volume of solution. This allows for accurate comparisons between different experiments and ensures consistency across studies.
Another advantage is that micromolar and molar units are widely recognized and understood within the scientific community. Researchers from various disciplines can easily interpret results reported in these units, facilitating collaboration and communication among scientists.
Additionally, using micromolar and molar concentrations allows for precise measurements, which is crucial when working with small amounts of substances or studying reactions with high specificity. The use of these units enables researchers to accurately determine the concentration at which certain biological processes occur.
However, it is important to note some limitations when using micromolars and molars. One limitation is that these concentration units may not be suitable for all types of experiments or substances. For instance, extremely low concentrations might require the use of picomolars or femtomolars instead.
Furthermore, variations in experimental conditions such as temperature or pH can affect the accuracy and reliability of measurements expressed in micromolar or molar concentrations. It is essential to account for these factors while interpreting data obtained using these units.
In conclusion (not conclusive), understanding the advantages and limitations associated with using micromolars and molars helps researchers make informed decisions about their experimental design. By considering factors such as sensitivity requirements, substance properties, and potential sources of error, scientists can choose the most appropriate concentration unit for their specific needs
Conclusion: Choosing the Right Unit for Your Research Needs
Choosing the Right Unit for Your Research Needs
In scientific research, precision and accuracy are of utmost importance. When it comes to concentration measurements, choosing the right unit can make all the difference in obtaining reliable data. Both micromolar and molar units have their advantages and limitations, so it’s essential to consider your specific research needs before making a decision.
Micromolars provide a more precise measurement for low-concentration substances due to their smaller scale. They are commonly used when analyzing small molecules or studying cellular processes where even slight changes in concentration can have significant effects. On the other hand, molar units offer simplicity and ease of calculation for larger concentrations. They are often employed when working with bulk solutions or conducting experiments on a macroscopic level.
To choose between micromolars and molar units, consider factors such as the nature of your samples, sensitivity requirements, experimental conditions, and intended applications. Ask yourself whether you need high precision at lower concentrations or if simplicity is essential for your study objectives.
Additionally, consult existing literature within your field to determine which unit is most commonly used by researchers in similar studies. Consistency with established standards can facilitate collaboration and comparison among different laboratories.
Remember that accurate measurements depend not only on selecting the appropriate unit but also on employing proper techniques and calibration methods during experimentation. Always strive for consistency throughout your work to ensure reproducibility.
There is no one-size-fits-all answer when it comes to choosing between micromolar and molar units—it depends entirely on your unique research needs. By carefully evaluating these needs alongside considerations of precision versus simplicity, you can confidently select the right unit for achieving meaningful results in your scientific endeavors.
So next time you embark on an experiment requiring concentration measurements—whether it be investigating biochemical pathways or assessing drug efficacy—take a moment to reflect on which unit will serve you best: micromolars or mols? The choice is yours!